Goal: When you have finished this laboratory exercise you will understand
- the sorption (adsorption and desorption) phenomena in food products
and you will learn
- how to determine the adsorption and desorption isotherms of yellow dent corn at different temperatures, and
- how to apply the GAB equation to experimental data to evaluate the adsorption and desorption isotherms.
-
The relationship between equilibrium moisture content and equilibrium relative humidity (water activity) is required in designing drying, storage and handling systems for agricultural products.
Moisture is adsorbed and desorbed at different rates by different foods or by the same food product under different conditions (e.g., temperature, relative humidity).
Therefore, there is a considerable interest in characterizing the entire sorption process for better designing and optimization of industrial processes. Sorption isotherm of a product describes its ability to adsorb or release water vapor from or into the surrounding atmosphere until an equilibrium state is reached.
-
Yellow dent corn with an initial moisture content of 12.79 kg water/kg dry matter and 0.698 water activity will be used in this experiment.
For adsorption isotherm, about 50 gram sample of corn will be initially placed in a small dish. The dish will be located on a platform inside a glass jar. Calcium sulfate will be placed at the bottom of the jar. The corn sample will be kept in this jar until there is no change in its weight. Then calcium sulfate will be replaced with a saturated salt solution to obtain desired equilibrium relative humidity in the jar. The jar will be placed inside a controlled temperature incubator. The sample will be kept in the jar until there is no change in its weight indicating equilibration with the surrounding atmosphere. The final weight of the sample will be recorded.
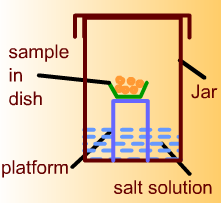
-
For desorption isotherm, about 50 gram sample of corn will be initially placed in a small dish. The dish will be located on a platform inside a glass jar. Distilled water will be placed at the bottom of the jar. The corn sample will be kept in this jar until there is no change in its weight. Then distilled water will be replaced with a saturated salt solution to obtain desired equilibrium relative humidity in the jar. The jar will be placed inside a controlled temperature incubator. The sample will be kept in the jar until there is no change in its weight indicating equilibration with the surrounding atmosphere. The final weight will be recorded.

-
By definition, water activity (aw) is the equilibrium relative humidity (ERH) in the surrounding environment of a food divided by 100.
The relationship between aw and equilibrium moisture content (EMC) of a food material at constant temperature and pressure is represented by its sorption isotherm.
Sorption isotherm of a product describes its ability to adsorb or release water vapor from or into the surrounding atmosphere until an equilibrium state is reached. -
For most food products, the relationship between moisture content and water activity is:
-
Different mathematical models have been applied to the experimental data to express the water sorption process of food products. Among the given models in the literature, the GAB equation is:
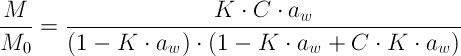
The GAB model describes the moisture content as a function of water activity ranging from 0 to 0.9.
In this experiment, we will determine the sorption isotherms of yellow dent corn at different temperatures and use the experimental data to calculate the GAB equation parameters.
Operator's Panel
Virtual Experiment
To obtain numerical data for further analysis, conduct this virtual experiment at www.rpaulsingh.com.
-
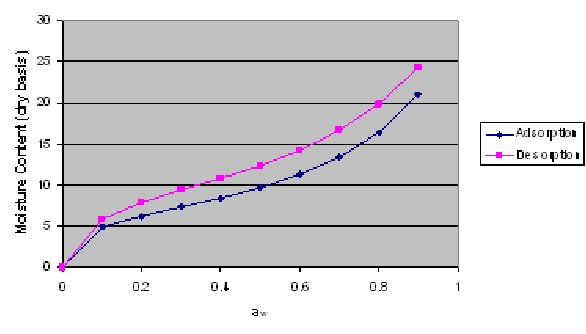
In this laboratory exercise, you obtained data on the change in moisture content vs water activity at different temperatures for yellow dent corn. We will now use the following procedure to determine the GAB equation parameters.
1. After obtaining the experimental data in Excel, the first step will be to reproduce the data in the next column (to prevent discontinuity, we will not use the first data point).
2. The next step is to plot the change in aw/M versus aw to determine the coefficients of the following parabolic equation:
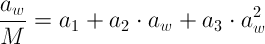
-
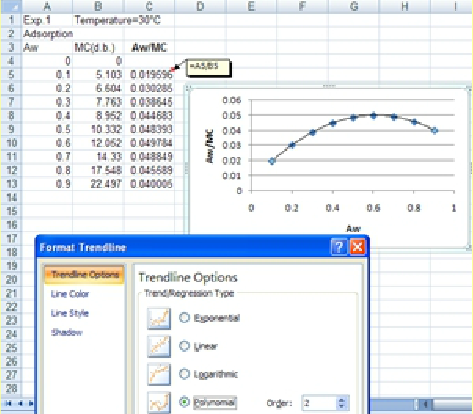
3. TrendLine option of Excel for a 2nd degree polynomial will be applied to determine the coefficients of the GAB equation.
-
The TrendLine option gives the required parameters of a1, a2 and a3 (see the figure on the following page) to determine the GAB equations parameters of K, C and M0 using the following equations:
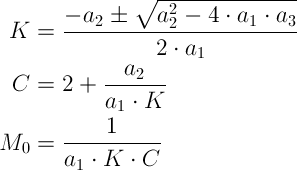
-
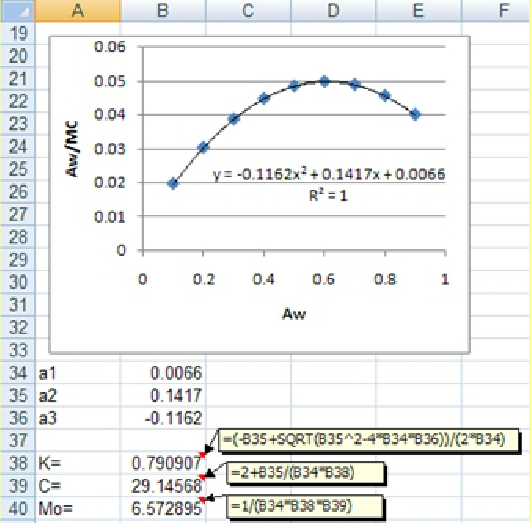
According to the given trendline in the above figure, the a1, and a2 and a3 values are found to be -0.1162, 0.1417 and 0.0066, respectively. Using these values, K, C and M0 values are easily calculated (see the previous page).
- How did K, C and M0 values change with temperature?
- What is the significance of the change of the monolayer moisture content with temperature?
- In addition to the GAB equation, there are several other empirical models used to describe data obtained for sorption isotherms of foods. Review published literature on this topic and list three different models that are commonly employed for this purpose.
- Anderson, R.B. (1946). Modification of the Branauer, Emmet and Teller equations. J. American Chem. Soc. 68:686-691.
- Bizot, H. (1983). Using the GAB model to construct sorption isotherms. In: Physical Properties of Foods ed. R. Jowitt, F. Escher, B. Hallstrom, H. Th. Meffert, W.E.L. Spiess and G. Vos. Applied Science Publishers, London.
- Branauer, S., Deming, L.S., Deming, W.E., and Teller, E. (1940). On the theory of the van der Walls adsorption of gases. J. American Chem. Soc.. 62:1723-1732.
- Chen, C. and Jayas, D.S. (2001). Evaluation of the GAB equation for the isotherms of agricultural products. Trans. ASAE. 41:1755-1760.
- De Boer, J.H. (1953). The dynamic character of adsorption. Clarendon Press, Oxford, England.
- Labuza, T.P. (1984). Moisture sorption: practical aspects of isotherm measurement and use. St. Paul, MN, American Society of Cereal Chemists.
- Peng, G., Chen, X., Wu, W. and Jiang, X. (2007). Modeling of water sorption isotherm of corn starch. J. Food Eng. 80:562-567.
- Samapundo, S., Devlieghere, F., De Meulenaer, B., Atukwase, A., Lamboni, Y. andd Debevere, J.M. (2007). Sorption isotherms and isoteric heats of sorption of whole yellow dent corn. J. Food Eng. 79:168-175.
- Van den Berg, C. (1984). Description of water activity of foods for engineering purposes by means of the GAB model of sorption. In:Engineering and Foods, Vol.1, ed. by B.M. McKenna. Elsevier Applied Science, New York, N.Y.